uAIFI Technology
The uAIFI Technology will provide the uMR® 680 with significant performance enhancements, more powerful imaging capabilities, better workflow, and improved user experience.

Experience the New Clinical Reality
The uMR®680 was designed with a high performance system: outstanding gradient performance of 45 mT/m & 200 T/m/s and excellent magnetic field homogeneity with a 70cm wide bore, expanding your capabilities in the field of clinical applications and research. Empowered by uAIFI Technology, the uMR®680 delivers '3.0T-like' image quality and achieves a new standard in workflow and patient care.
Facilitates clinical applications and research
45 mT/m & 200 T/m/s
High Performance Gradient System
Simultaneously improves SNR and image sharpness
uAIFI DeepRecon: Provides 1.5T System with '3.0T-like' Image Sharpness
Streamlines the workflow and provides more comfort
uAIFI EasySense: Contactless Sensing of Patients' Respiratory Motions


Powerful Gradient Performance
The remarkable 45 mT/m & 200 T/m/s (simultaneous on each axis) unlock higher signal-to-noise ratio (SNR) for neuro and cardiac imaging.
High-Density RF Channels
Enable the full use of high-density surface coil arrays, thus significantly improving image quality.

Intelligently removes noise from images while maintaining excellent diagnostic image quality.
Delivers high-resolution anatomical structure with sharper anatomical structure.
A double safety lock design in both image domain and K-space domain for high-reliability results.
Applicable for multi-bed scanning such as whole spine and whole-body imaging.

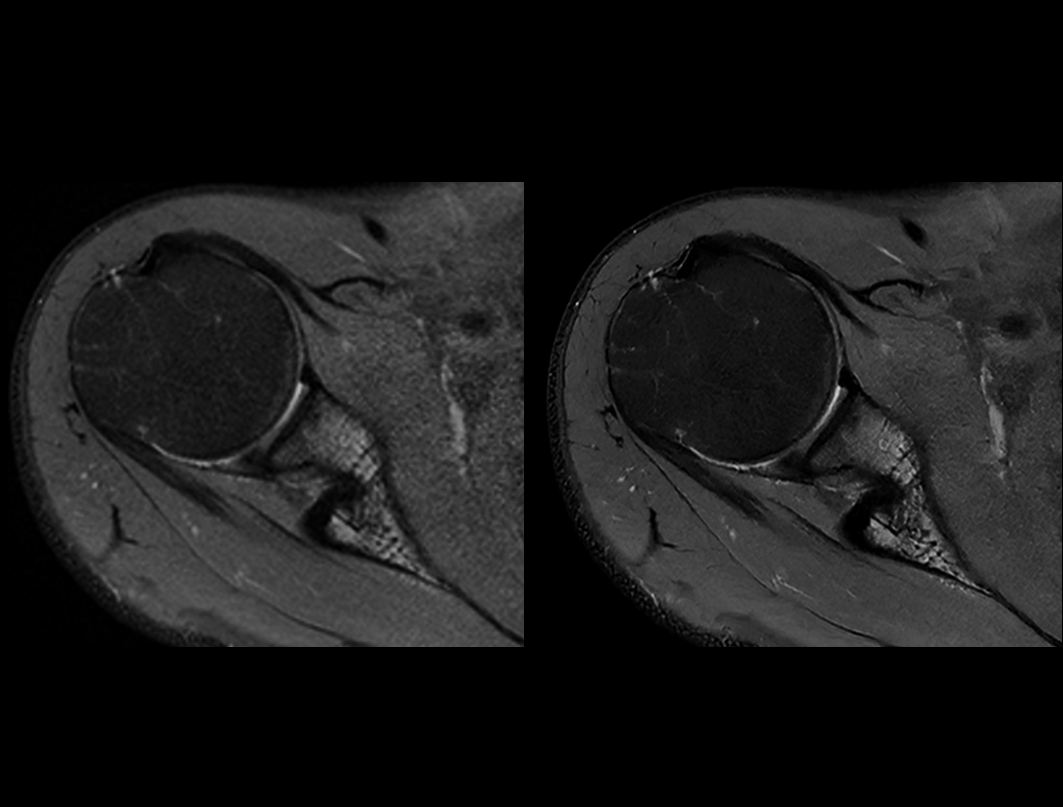
Shoulder PDW FS
Conventional (2:56 min) vs. uAIFI DeepRecon™ (2:56 min)
0.29×0.29×3 mm3
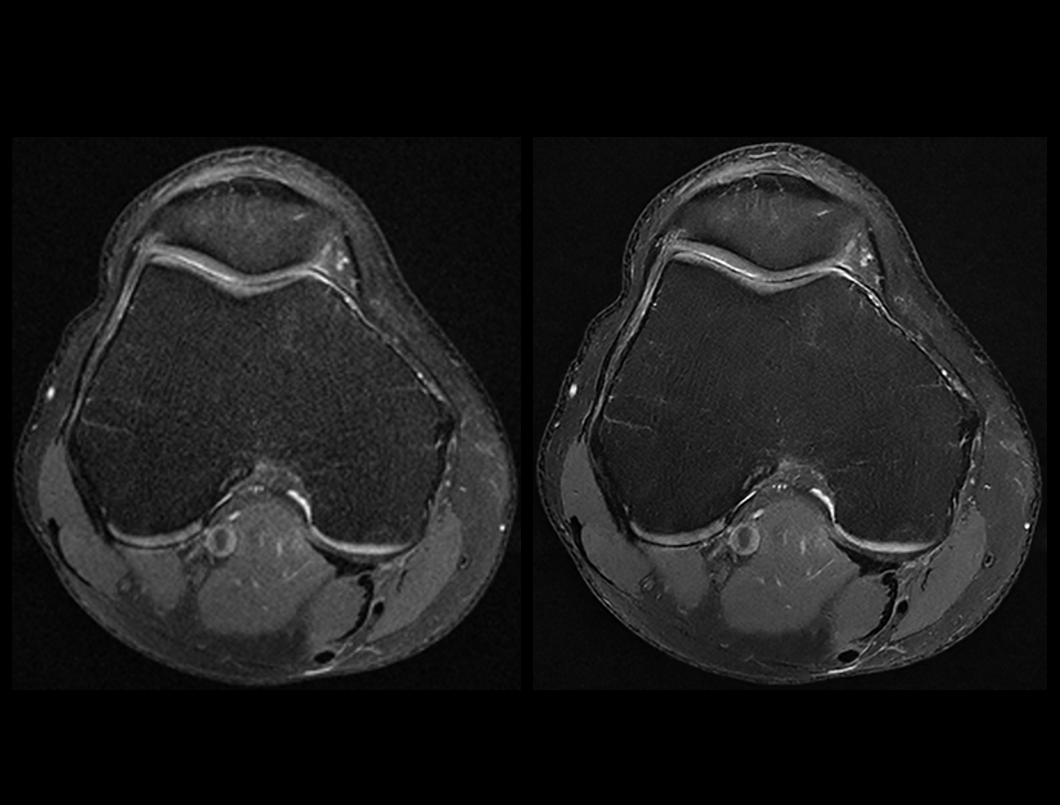
Knee PDW FS
Conventional (2:32 min) vs. uAIFI DeepRecon™ (2:32 min)
0.35×0.35×3 mm3
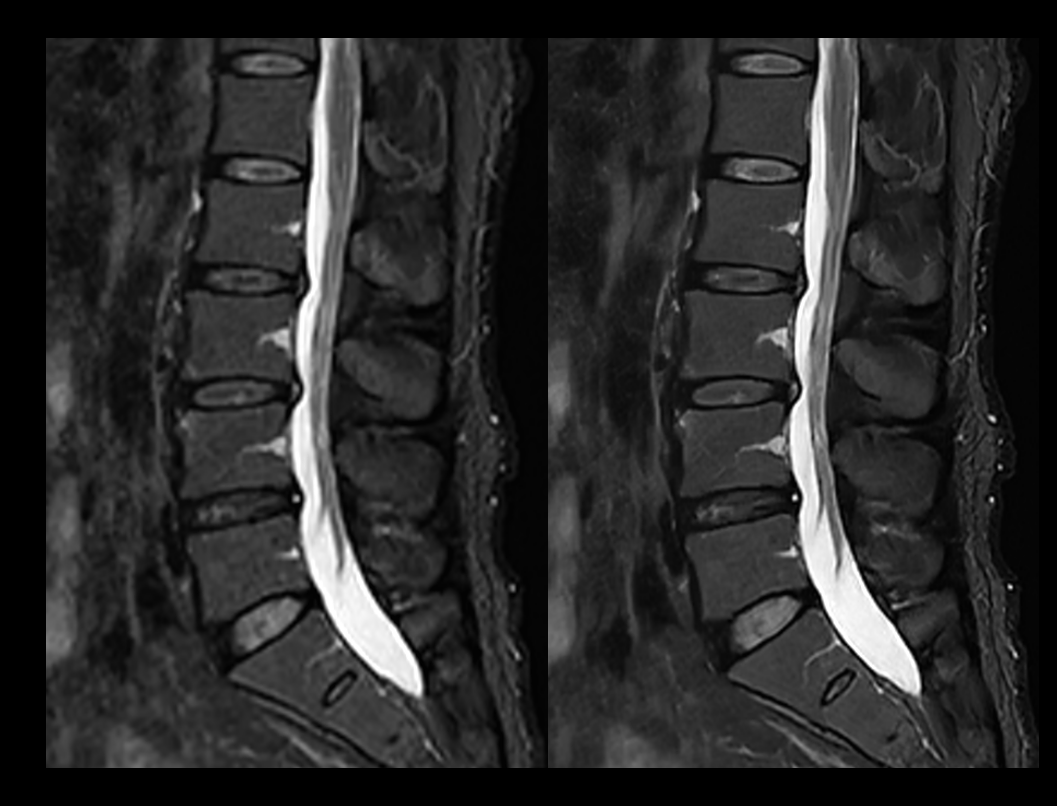
Spine T2W STIR
Conventional (2:44 min) vs. uAIFI DeepRecon™ (2:44 min)
1.11×1.11×4 mm3
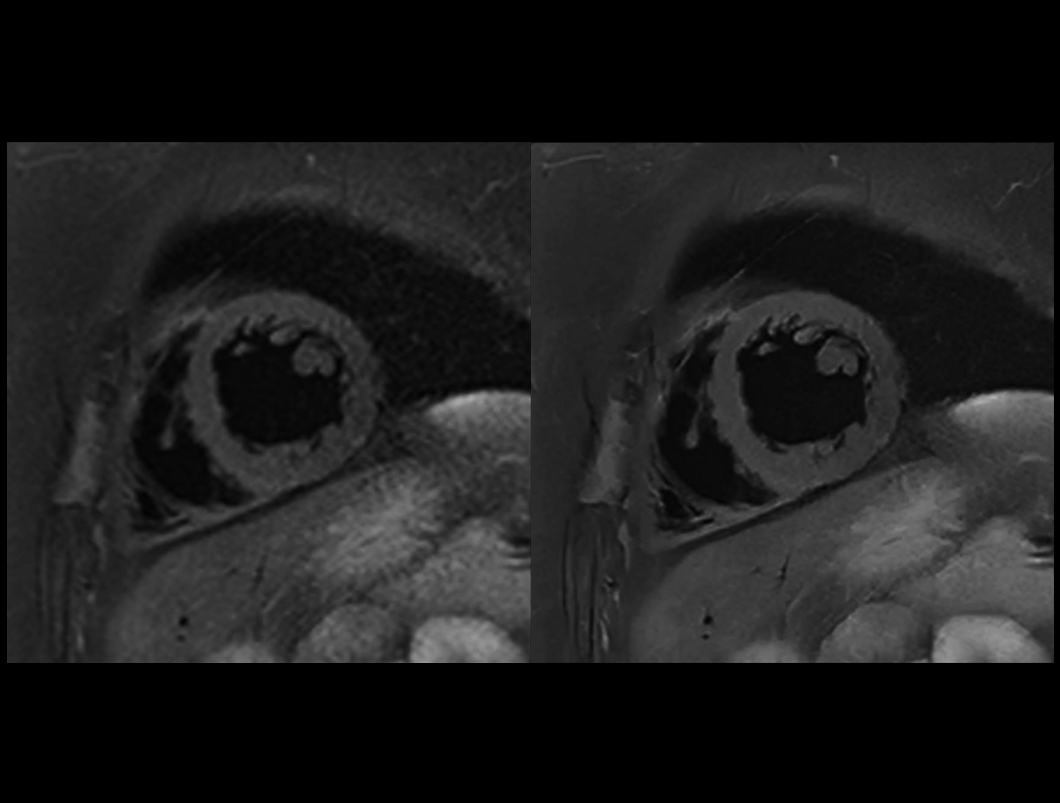
Cardiac T2W DB SPAIR
Conventional (9.4 sec) vs. uAIFI DeepRecon™ (9.4 sec)
0.78×0.78×4 mm3
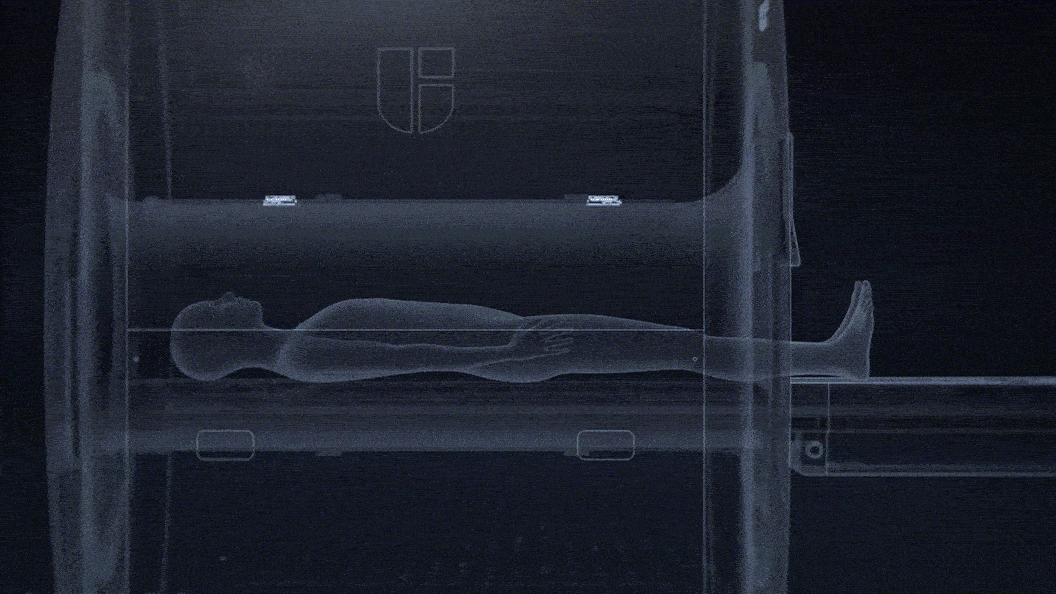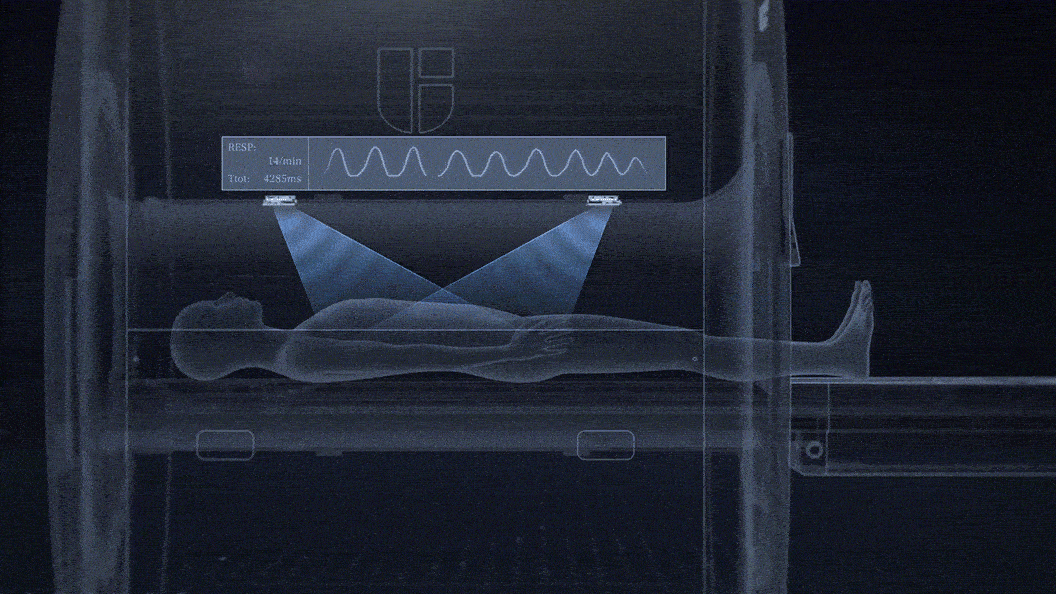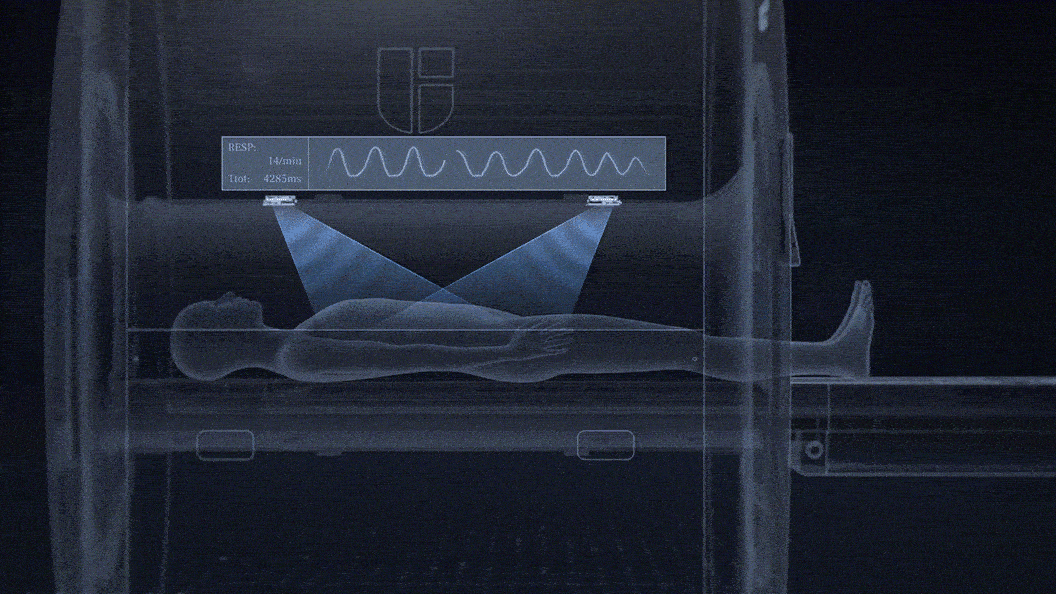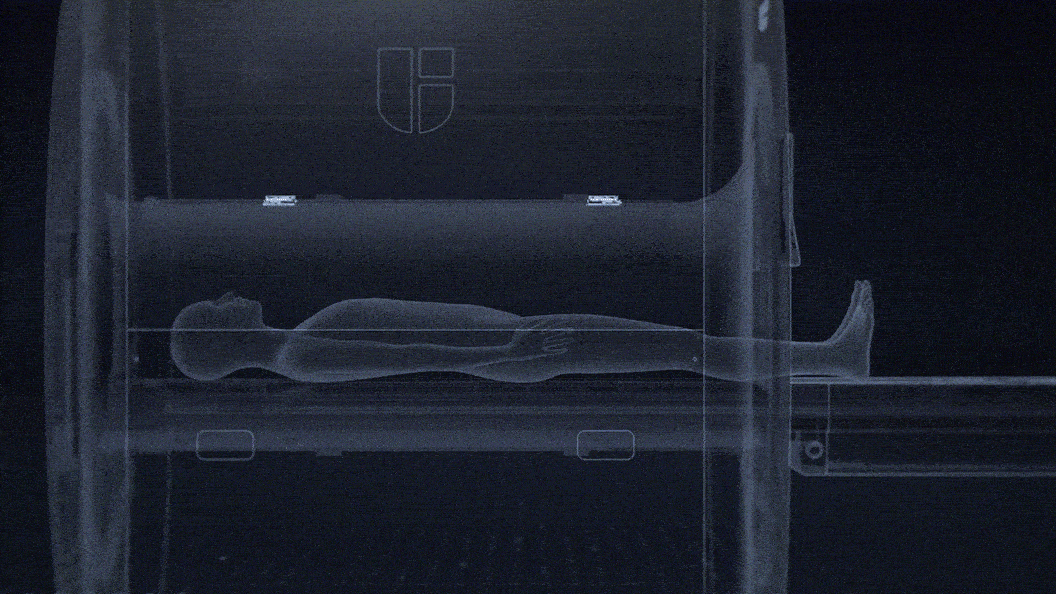
No need for technologists to attach a respiratory belt to patients.
Contactless sensing for free breathing, better for patients with respiratory challenges.
Fully integrated within the MRI bore and unobstructed by clothing or MRI RF coils.
0.1mm detection accuracy of breathing motion with a temporal resolution up to 20ms.
